Abstract
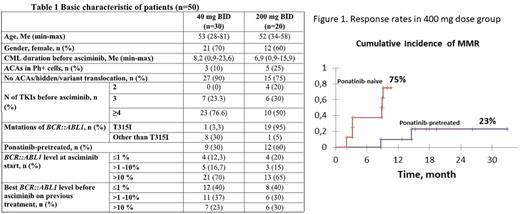
Background: Asciminib is a Specifically Targeting the ABL Myristoyl Pocket (STAMP) ABL. kinase inhibitor that has shown efficacy and a good safety profile in clinical trials in patients with Ph-positive chronic myeloid leukemia (CML) failing prior TKI lines. It was confirmed by real world data in Novartis approved Managed Access Program (MAP) in several countries.Aim: to present two-year updated results of asciminib use in clinical practice in the MAP program in 3 centers of Russia. Methods: In total the data of 50 CML pts who received asciminib for more than 9 months and did not undergo bone marrow transplantation was analyzed. Pts recruitment, dosing regimen, response monitoring, and toxicity control were performed according to the MAP treatment plan. All pts had a chronic phase (CP) CML, 8 pts had a second CP after accelerated phase (n=7) or blast crisis (BC). Complete cytogenetic response (CCyR) corresponding to BCR::ABL1 ≤1%, major molecular response (MMR) and deep molecular response (MR4) achievement was assessed by cumulative incidence function (CIF) with Gray's test for comparison responses in subgroups. Survival analysis was performed by Kaplan-Meier method. Results: All pts were divided into two groups: 20 pts received an initial dose of 200 mg BID (19/20 pts with BCR::ABL1 T315I ), 30 pts received 40 mg BID (1/30 pts with BCR::ABL1 T315I ). Baseline characteristics of the two dose groups are presented in table 1. The median follow-up among all pts was 17 months (range 10-37); 7(14%) pts discontinued asciminib (5 due to resistance, 2 due to progression). Two-year survival rate without treatment discontinuation was 83%. The 2-year OS was 100% and 93% in 400 mg and 80 mg dose groups, respectively, two (4%) pts progressed to BC and died. CIF of CCyR, MMR and MR4 by 24 month was 55%, 29% and 17% for 80 mg dose and 45%, 48% and 45% for 400 mg dose respectively. There was no significant difference in response rates between the two dose groups. In the 400 mg dose group, the cumulative incidence of MMR was significantly higher in ponatinib-naive patients (75% vs. 23% in ponatinib-pretreated patients, p=0.006) (figure 1). Twenty two (44%) pts experienced any adverse events (AEs) and 8 (16%) had AEs of grade 3-4. AEs of grade 3-4 connected with asciminib included neutropenia, thrombocytopenia and irregular menstruation. No pts stopped treatment due to toxicity; no difference in toxicity was found between doses of 80 and 400 mg/day. Conclusion: Asciminib shows promising results for the treatment of highly pre-treated CML pts and should be considered as therapeutic options for pts with failure to previous TKIs. The 400 mg dose was well tolerated and highly effective in ponatinib-naive pts with BCR::ABL1 T315I and should be investigated in clinical trials in patients without T315I as a potentially more effective and well tolerated dose.
Disclosures
Turkina:Novartis: Other: Travel, Accommodation, Expenses , Speakers Bureau; Pfizer: Other: Travel, Accommodation, Expenses , Speakers Bureau; Fusion Pharma: Speakers Bureau. Lomaia:Pfizer: Other: Travel, Accommodation, Expenses , Speakers Bureau; Bristol Myers Squibb: Other: Travel, Accommodation, Expenses ; Fusion Pharma: Speakers Bureau; Novartis: Other: Travel, Accommodation, Expenses , Speakers Bureau. Morozova:Pfizer: Honoraria, Speakers Bureau; AMGen: Speakers Bureau; Novartis: Speakers Bureau. Shukhov:Novartis: Consultancy, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau. Petrova:AMGen: Speakers Bureau. Vlasova:Pfizer: Speakers Bureau; Novartis: Speakers Bureau. Chelysheva:Pfizer: Speakers Bureau; Novartis Pharma: Speakers Bureau. Sbityakova:AstraZeneca: Speakers Bureau; Novartis: Speakers Bureau. Gurianova:Pfizer: Speakers Bureau. Kokhno:Novartis: Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.